Yasunori Hayashi: Difference between revisions
yhlabe>Yasunori Hayashi |
m (1 revision imported) |
(No difference)
| |
Latest revision as of 19:30, 11 April 2023
Yasunori Hayashi was born on July 28, 1965 in Nagoya, Aichi Prefecture and grew up in Tokyo.
Education and Research History
- 1984 - 1990 Kyoto University Faculty of Medicine, MD
- 1990 - 1994 Institute for Immunology (Prof. Shigetada Nakanishi) and Department of Pharmacology (Prof. Shuh Narumiya), Kyoto University Faculty of Medicine, PhD
- 1994 - 1996 Postdoctral Fellow, Department of Neurophysiology (Prof. Tomoyuki Takahashi), Institute for Brain Research, Faculty of Medicine, University of Tokyo
- 1996 - 2000 Postdoctral Fellow, Cold Spring Harbor Laboratory (Dr. Roberto Malinow)
- 2000 - 2009 Assistant Professor (joint), RIKEN-MIT Neuroscience Research Center, The Picower Institute for Learning and Memory, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology
- Senior Scientist (joint), Brain Science Institute, RIKEN
- 2004 - 2009 Unit Leader (joint), Brain Science Institute, RIKEN
- 2009 - 2013 Team Leader, Brain Science Institute, RIKEN
- 2013 - 2017 Senior Team Leader, Brain Science Institute, RIKEN
- 2016 - Professor Kyoto University Graduate School of Medicine, Department of Pharmacology
Past Accomplishments
- Please see project page for recent accomplishments.
Regulation of Adrenal Tyrosine Hydroxylase under Hypoxic Condition
The three catecholamines, dopamine, noradrenaline, and adrenaline, are produced by a biosynthetic pathway whose rate-limiting step is tyrosine hydroxylase, which catalyzes reaction to produce dopa from tyrosine and oxygen. In the adrenal medulla, catecholamines are released in response to stress. We therefore investigated how catecholamine synthesis is regulated under hypoxia stress[1- 1]. Catecholamine synthesis decreased in the brain, but increased in the adrenal glands. This is likely a response to the increased demand for catecholamines due to hypoxic stress, but the question is how to compensate for the decrease in substrate, oxygen. I found that the increased affinity for substrate tyrosine as well as an increase in the concentration of tyrosine in the tissue compensates the reduced availability of oxygen. This research will help us understand how neurons reacts to hypoxic conditions, such as cerebral ischemia.
- ↑
Hayashi, Y., Miwa, S., Lee, K., Koshimura, K., Hamahata, K., Hasegawa, H., Fujiwara, M., & Watanabe, Y. (1990).
Enhancement of in vivo tyrosine hydroxylation in the rat adrenal gland under hypoxic conditions. Journal of neurochemistry, 54(4), 1115-21. [PubMed:1968954] [WorldCat] [DOI] [Google Scholar]
Cloning and Functional Analysis of Prostanoid Receptor
Prostanoids include prostaglandins and other arachidonic acid metabolites. Based on the partial sequence of thromboxane A2 receptor protein purified by Narumiya Laboratory at Kyoto University School of Medicine, we cloned the full-length cDNA from a human placental cDNA library[2- 1][2- 2]. We then analyzed the expression of the protein in Xenopus oocytes expression system. In addition, using the thromboxane A2 receptor sequence, we searched for other subtype receptors and cloned the EP3 subtype of the prostaglandin E2 receptor and contributed to the functional analysis[2- 3][2- 4][2- 5].
- ↑
Hirata, M., Hayashi, Y., Ushikubi, F., Yokota, Y., Kageyama, R., Nakanishi, S., & Narumiya, S. (1991).
Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature, 349(6310), 617-20. [PubMed:1825698] [WorldCat] [DOI] [Google Scholar] - ↑
Namba, T., Sugimoto, Y., Hirata, M., Hayashi, Y., Honda, A., Watabe, A., Negishi, M., Ichikawa, A., & Narumiya, S. (1992).
Mouse thromboxane A2 receptor: cDNA cloning, expression and northern blot analysis. Biochemical and biophysical research communications, 184(3), 1197-203. [PubMed:1375456] [WorldCat] [DOI] [Google Scholar] - ↑
Negishi, M., Sugimoto, Y., Hayashi, Y., Namba, T., Honda, A., Watabe, A., Narumiya, S., & Ichikawa, A. (1993).
Functional interaction of prostaglandin E receptor EP3 subtype with guanine nucleotide-binding proteins, showing low-affinity ligand binding. Biochimica et biophysica acta, 1175(3), 343-50. [PubMed:8382086] [WorldCat] [DOI] [Google Scholar] - ↑
Sugimoto, Y., Namba, T., Honda, A., Hayashi, Y., Negishi, M., Ichikawa, A., & Narumiya, S. (1992).
Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. The Journal of biological chemistry, 267(10), 6463-6. [PubMed:1372606] [WorldCat] [Google Scholar] - ↑
Sugimoto, Y., Negishi, M., Hayashi, Y., Namba, T., Honda, A., Watabe, A., Hirata, M., Narumiya, S., & Ichikawa, A. (1993).
Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. The Journal of biological chemistry, 268(4), 2712-8. [PubMed:8381413] [WorldCat] [Google Scholar]
Pharmacological Analyses of Metabotropic Glutamate Receptor
Metabotropic glutamate receptors (mGluRs) were first cloned in Nakanishi Laboratory. The cloning of mGluRs revealed that they form a group of structurally and functionally distinct receptors. It was therefore important to understand the physiological functions of each subtype based on molecular diversity. However, the subtype-specific drugs, crucial for understanding the role of each subtype were lacking. Therefore, we decided to cscreen for drugs using this cell line by establishing a cell line that stably expresses the cloned receptor in CHO cells. As a result, we found that L-CCG-I and DCG-IV, both carboxycyclopropylglycine derivatives, have subgroup 2 mGluR-specific agonist activity[3- 1][3- 2]. In addition, we found that MCPG, a phenylglycine derivative, has subgroup 1 mGluR and subgroup 2 specific antagonist activity[3- 3][3- 4].
Next, we examined the role of mGluR2 in accessory olfactory bulb signaling. The mitral cells of the accessory olfactory bulb form bidirectional dendrodendritic synapses with granule cells. The granule cells are excited by the glutamate released from the mitral cells, while the mitral cells are inhibited by GABA. Histological observations show that mGluR2 protein is localized on the granule cell side of the interdendritic synapse. Therefore, we activated mGluR2 by DCG-IV and examined the effect on GABA transmission from granule cells to mitral cells electrophysiologically. As a result, we found that glutamate released from mitral cells suppressed the release of GABA when it acted on granule cells, and the inhibition on mitral cells was released[3- 2]. This mechanism is thought to be a lateral inhibition mechanism via granule cell dendrites that improves the signal-to-noise ratio and enhances discrimination of olfactory input.
We further explored the function of mGluR2 in the accessory olfactory bulb from a behavioral perspective. It is known that female mice memorize the odor of their male mates during mating and then aborts pregnancy when they detects a male of an unknown strain (Bruce effect). Using this behavioral approach, we investigated how activation of mGluR2 by DCG-IV injection into the accessory olfactory bulb affects memory formation[3- 5]. In combination with the electrophysiological results showing that DCG-IV inhibits GABA release, this may be the result of mGluR2 activation decreasing inhibitory input to mitral cells and increasing excitability. In addition to the electrophysiological results showing that GABA release was inhibited by mGluR2, the activation of mGluR2 was thought to be the result of decreased inhibitory input to mitral cells and increased excitability.
- ↑
Hayashi, Y., Tanabe, Y., Aramori, I., Masu, M., Shimamoto, K., Ohfune, Y., & Nakanishi, S. (1992).
Agonist analysis of 2-(carboxycyclopropyl)glycine isomers for cloned metabotropic glutamate receptor subtypes expressed in Chinese hamster ovary cells. British journal of pharmacology, 107(2), 539-43. [PubMed:1330184] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑ 2.0 2.1
Hayashi, Y., Momiyama, A., Takahashi, T., Ohishi, H., Ogawa-Meguro, R., Shigemoto, R., Mizuno, N., & Nakanishi, S. (1993).
Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature, 366(6456), 687-90. [PubMed:7903116] [WorldCat] [DOI] [Google Scholar] - ↑
Hayashi, Y., Sekiyama, N., Nakanishi, S., Jane, D.E., Sunter, D.C., Birse, E.F., Udvarhelyi, P.M., & Watkins, J.C. (1994).
Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. The Journal of neuroscience : the official journal of the Society for Neuroscience, 14(5 Pt 2), 3370-7. [PubMed:8182479] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑
Sekiyama, N., Hayashi, Y., Nakanishi, S., Jane, D.E., Tse, H.W., Birse, E.F., & Watkins, J.C. (1996).
Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes. British journal of pharmacology, 117(7), 1493-503. [PubMed:8730745] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑
Kaba, H., Hayashi, Y., Higuchi, T., & Nakanishi, S. (1994).
Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science (New York, N.Y.), 265(5169), 262-4. [PubMed:8023145] [WorldCat] [DOI] [Google Scholar]
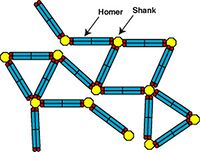
Structural Framework of Postsynaptic Density
Postsynaptic density (PSD) is an electron-dense structure beneath the synapse. But how it retains its structure and constituent proteins has not been known. We found that long splice-variants of Homer forms a tetramer through its coiled-coil domain and cross-links Shank[4- 2][4- 1] , thereby forming mesh-like structure. In contrast, the short-form of Homer is activity-dependently translated and works as a dominant negative form to destroy this structure[4- 3] , thereby working as a natural dominant negative form to homeostatically regulate synaptic transmission[4- 4].
- ↑ 1.0 1.1
Hayashi, M.K., Tang, C., Verpelli, C., Narayanan, R., Stearns, M.H., Xu, R.M., Li, H., Sala, C., & Hayashi, Y. (2009).
The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell, 137(1), 159-71. [PubMed:19345194] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑
Hayashi, M.K., Ames, H.M., & Hayashi, Y. (2006).
Tetrameric hub structure of postsynaptic scaffolding protein homer. The Journal of neuroscience : the official journal of the Society for Neuroscience, 26(33), 8492-501. [PubMed:16914674] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑
Sala, C., Futai, K., Yamamoto, K., Worley, P.F., Hayashi, Y., & Sheng, M. (2003).
Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. The Journal of neuroscience : the official journal of the Society for Neuroscience, 23(15), 6327-37. [PubMed:12867517] [PMC] [WorldCat] [DOI] [Google Scholar] - ↑
Hayashi, Y., Okamoto, K., Bosch, M., & Futai, K. (2012).
Roles of neuronal activity-induced gene products in Hebbian and homeostatic synaptic plasticity, tagging, and capture. Advances in experimental medicine and biology, 970, 335-54. [PubMed:22351063] [WorldCat] [DOI] [Google Scholar]
Awards and Honors
- 1998 Young Investigator Award from Japanese Pharmacological Society
- 2006 Departmental Teaching Award
- 2008 JSPS Prize for Young Investigators
- 2008 Japan Academy Medal
- 2019 Toshihiko Tokizane Memorial Award
Societies
- Society for Neuroscience
- The Japan Neuroscience Society
- The Japanese Pharmacological Society
- The Japanese Physiological Society
Personal Aspects
- Yasunori's favorite activity is listening music and train ride. His favorite music composer is Johann Sebastian Bach. He especially favors pipe organ musics by Bach.
- His favorite author is a novelist Akira Yoshimura. He also likes essays by Masahiko Fujiwara.
Contact
Department of Pharmacology
Kyoto University Graduate School of Medicine
Room 401, Building A
Kyoto 606-8501 Japan
Tel +81-75-753-7531 x 84393
E-Mail : yhayashi-tky@umin.ac.jp
- Please limit your inquiry only to topics related to our research and education program.
- We respectfully decline all consultations on health issues of yourself or of others.
External links
- Google Scholar (citation metrics)
- ResearcherID (citation metrics)
- Research Map
 https://orcid.org/0000-0002-7560-3004
https://orcid.org/0000-0002-7560-3004