Erasing memory with light -Understanding why sleep is necessary for good memory-
Outline
In an American movie “Men in Black”, Agent J, portrayed by Will Smith, erases the memories of people who have witnessed aliens by irradiating light from a secret devise, the Neuralizer. Research by a group led by Assistant Professor Akihiro Goto and Professor Yasunori Hayashi at the Graduate School of Medicine showed this is no longer a sci-fi.
They employed a fluorescent protein derived from sea anemones to accomplish this. Upon illumination, this protein releases reactive oxygen species (ROS), which then inactivates the surrounding proteins. They connected this protein with a synaptic protein important for memory. Using it, they were able to erase only the synapses that retained the memory.
Next, by introducing this protein into various parts of the brain, they were able to erase memories with light. Interestingly, they found that there are synapses in different parts of the brain that can remember immediately after learning, during sleep, and during sleep the next day. This research has contributed to the advancement of memory and sleep research by proposing a new cellular model of the function of sleep that maintains memory for a long time. The results of this research were published online in the international academic journal “Science” on October 2021.
Background
Memories are initially formed in the hippocampus for a short period of time, then subsequently transferred to the cortex for a long-term storage. This phenomenon is called "memory consolidation," but the cellular activity responsible for it has not been fully elucidated. Long-term potentiation (LTP) of synaptic transmission, which increases the efficiency of the transmission of neural activity between cells for long-term, is known as a cellular phenomenon of memory. It is believed that memories are formed in cells in which LTP is induced. Therefore, it is possible to determine when and in which cells memories are retained by examining the time and cells in which LTP is induced during the process of memory consolidation. However, until now there has been no technique to examine this. Therefore, this study was started from developing a technique to detect when and where LTP is occurring, and using this technique to identify the cells and time window in which LTP is induced during memory consolidation.
Methods
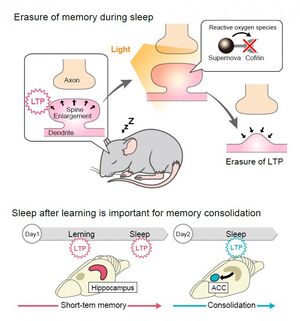
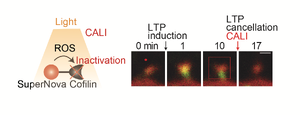
In order to detect the time window in which LTP occurs, we have developed a method to erase LTP by light. sLTP (structural LTP) is known to be accompanied by LTP, in which the postsynaptic spine structure enlarges. Since cofilin, an actin-binding protein, is important for the enlargement of the spine, we developed a technique for light-induced erasure of sLTP and LTP by inactivating cofilin using a technique called CALI (Fig. 1). For this purpose, we used SuperNova, a fluorescent protein from a sea anemone. SuperNova releases reactive oxygen species (ROS) to inactivate surrounding proteins when exposed to light. When SuperNova is irradiated with light, it releases ROS that inactivate surrounding proteins, and when cofilin was inactivated using this property, LTP was erased. Although we could prevent induction of LTP using drugs in the past, this technology using light made it possible for the first time to erase LTP only at a specific time window.
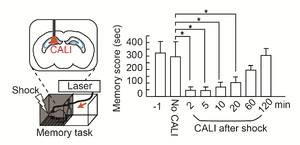
Using this novel optogenetic tool, we found light irradiation into the hippocampus immediately after learning or during sleep after learning leads to erasure of the memories in both cases. This indicates that two waves of LTP occurred in the hippocampus immediately after learning and during sleep afterwards, and that these stepwise LTP events shaped short-term memories in the hippocampus (Fig. 2).
Furthermore, we found these two forms of LTP had distinct effects. By observing the activity of cells using calcium imaging, they found that LTP immediately after learning caused cells to specifically fire in a familiar context, and subsequent LTP during sleep caused cells to fire synchronously. Since it has been known that groups of memory cells exhibit synchronized and correlated firing (cell assemblies), this finding provides a more detailed view of the process of memory cell formation.
To further understand the time window in which memories are transferred to the cortex, we examined the time window of LTP in the anterior cingulate cortex, a cortical region implicated in the recall of old memory, and found that LTP was induced in the anterior cingulate cortex during sleep the day after learning but not on the same day. In other words, memories for long-term storage had already begun to be transferred to the cortex the day after learning.
Implications
In this study, we developed a technique to detect the time window in which LTP is induced, which is a common mechanism of memory formation not only in the hippocampus and cortex but also in many brain regions involved in memory. Therefore, this technology has the potential to elucidate many brain functions involved in memory at the cellular level. In addition, understanding the brain function of long-term memory retention, which is important in our daily lives, will lead to a better understanding of memory. So this work also has a social impact. It has also been suggested that synaptic abnormalities related to LTP are involved not only in memory and learning disorders such as developmental disorders, post-traumatic stress disorder (PTSD), dementia, and Alzheimer's disease, but also in the development of psychiatric diseases such as schizophrenia and depression. The findings of this study are expected to lead to a wide range of treatments for mental disorders.
Research Team
This research was conducted in collaboration with the RIKEN Brain Science Institute (team leader Masanori Murayama's group and Thomas McHugh's group) and the Institute of Scientific and Industrial Research, Osaka University (Professor Takeharu Nagai's group).
Funding
Grant-in-Aid for Scientific Research JP21650080, JP16H01292, JP16H01438, JP16H02455, JP17K19631, JP18H05434, JP19H01010, JP19H05233 , JP15K06728, JP17H05949, JP18K14818, JP20K15901, JP18H05410 from MEXT, Japan, Uehara Memorial Foundation for Life Science, Naito Memorial Foundation for the Advancement of Science, Foundation for the Advancement of Photonic Science and Technology, Novartis Science Foundation, Takeda Science Foundation, HFSP Research Grant (RGP0022/2013), JST CREST (JPMJCR20E4), JST PRESTO (JPMJPR0165 ), and Inamori Foundation.
Glossary
Long-term potentiation
Signal transmission between neurons is mediated by synapses. The efficacy of transmission can change according to the input pattern. This property is called synaptic plasticity. Long-term potentiation (LTP) is a representative form of synaptic plasticity. This sustained enhancement of signal transmission between neurons by LTP is thought to be the cellular level phenomenon of memory. This phenomenon is accompanied by structural change of synapse, which is known as sLTP (structural LTP).
CALI
CALI is a technology that uses light to inactivate specific proteins. In this study, a photosensitizing fluorescent protein called SuperNova was fused to cofilin, an actin-related molecule. When irradiated with light of a specific wavelength, SuperNova generates reactive oxygen species from the light energy and inactivates only nearby cofilin.
Words from the first author
Although LTP has long been proposed to underly memory, it’s importance in each brain region for memory consolidation has not been so much reported. Therefore, the findings that elimination of local LTP by light illumination leads to clear erasure of memory is surprising for me. Especially, the time window when it actually occurs in each brain region during memory formation remains elusive. Therefore, the findings that stepwise LTP events occur in hippocampus and anterior cingulate cortex, and such local LTP events are required for memory formation is interesting for me.
Publication information
-
Goto, A., Bota, A., Miya, K., Wang, J., Tsukamoto, S., Jiang, X., Hirai, D., Murayama, M., Matsuda, T., McHugh, T.J., Nagai, T., & Hayashi, Y. (2021).
Stepwise synaptic plasticity events drive the early phase of memory consolidation. Science (New York, N.Y.), 374(6569), 857-863. [PubMed:34762472] [WorldCat] [DOI] [Google Scholar]