An oil-water relationship explains memory formation - A new protein segregation mechanism in the brain
Outline
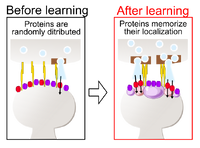
Humans built our civilization by memorizing knowledge and skills. We spend our daily lives memorizing the faces and events of our loved ones. Memory is a brain function that permanently stores information of temporary events, such as what we have seen or eaten. During this process, the molecules in the brain undergo certain changes that withstand their turnover over time beyond the lifetime of the molecules themselves. A research group led by Professor Yasunori Hayashi, Postdoctoral Fellow Tomohisa Hosokawa (at the time of the study, currently a lecturer at Nagoya University), and doctoral student Pinwu Liu at Kyoto University Graduate School of Medicine has shown that proteins in the brain form condensates when stimulated during learning. The neurons are filled with water, but these aggregates spontaneously separate and assemble from the water in the neurons as if they were oil droplets floating on water. The molecules themselves remember their own locations, while allowing them to turnover. In this way, this mechanism allows us to retain their memories throughout our lives. We hope our finding leads to a better understanding and eventually to a treat neurological diseases such as Alzheimer's disease.
Background
Although synaptic plasticity is thought to be a fundamental mechanism for learning and memory, the molecular mechanisms that allow synaptic plasticity such as long-term potentiation to occur instantaneously and persistently have remained unclear. In recent years, the assembly of proteins by liquid-liquid phase separation has attracted much attention as a novel principle of protein behavior in cells. In context of cell biology, liquid-liquid phase separation is a phenomenon where proteins and nucleic acids form condensates while retaining liquid-like properties in the cytoplasm. The resultant condensates are stable and segregated from the rest of cytoplasm without any demarcating lipid bilayer. It attracts attention as a novel mechanism to form membrane-free organelles and attracts attention. We consider the liquid-liquid phase separation might explain the the assembly of proteins seen during synaptic plasticity events, which requires both dynamics and persistence.
Research Methods and Results
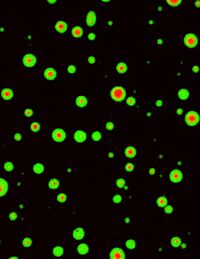
It is well established that influx of calcium ions by synaptic activity triggers memory formation. We hypothesized that calcium-dependent protein kinases play a central role in the assembly of synaptic proteins. We therefore purified the calcium-dependent protein kinases and representative synaptic proteins and then observed the effect of calcium ions in vitro. As a result we found the proteins underwent liquid-liquid phase separation and formed condensates (Fig. 1). The condensates persisted even after the calcium ions were removed. Interestingly, in the presence of multiple synaptic proteins, a phase-in-phase was formed in which additional proteins formed aggregates within the aggregates. This indicates that proteins alone can form intracellular structures with complex domain organization. The physiological significance of this assembly was investigated by observing synapses with a super-resolution microscopy. We suggest that the assembly enhances the efficiency of synaptic transmission by aligning the localization of postsynaptic proteins with that of presynaptic proteins (Fig. 2). These results provide a new molecular mechanism of synaptic plasticity and memory formation, which may greatly advance efforts to treat memory disorders such as Alzheimer's disease.
Implication and Future Perspectives
This study proposes a new molecular mechanism for the formation and maintenance of memory. It has been suggested that the separation and assembly of protein condensate by liquid-liquid phase separation can be controlled by small molecules and peptides, which may be applied to the establishment of therapies for diseases associated with memory impairment. It is also compatible with optogenetics, which uses light to control the activity and localization of proteins, and is expected to become a new tool for memory research. On the other hand, there are several hundred types of proteins that actually form synaptic assemblies, and without an understanding of the behavior of each of these proteins, it is not possible to fully understand the assembly formation as a molecular mechanism of memory. It is hoped that a comprehensive analysis method will be developed.
Research Team
This research was conducted in collaboration with Kyoto University, Hong Kong University of Science and Technology (Prof. Mingjie Zhang's group), and University of Bordeaux (Prof. Eric Hosy, Prof. Laurent Groc, Prof. Daniel Choquet, Prof. Jean-Baptiste Sibarita's group).
Research Support
This work was supported by RIKEN Presidents Fund, SPIRITS 2019 of Kyoto University, Grant-in-Aid for Scientific Research JP20240032, JP22110006, JP16H01292, JP18H04733 and JP18H05434 from the MEXT, Japan, JST, CREST JPMJCR20E4, Japan, Programme Exploration France from Ambassade de France au Japon, The Uehara Memorial Foundation, The Naito Foundation, Research Foundation for Opto-Science and Technology, Novartis Foundation, The Takeda Science Foundation and Japan Foundation for Applied Enzymology (Hayashi), The Takeda Science Foundation and Grants-in-Aid for Scientific Research JP17K14947, JP18KK0421 and JP19K06885 from the MEXT, Japan (Hosokawa), grants from the Simons Foundation (Award ID 510178) and Research Grant Council of Hong Kong (AoE-M09-12 and C6004-17G) to (Zhang), HFSP Research Grant (RGP0020/2019) jointly to (Hayashi and Zhang), CRCNS-NIH-ANR AMPAR-T fellowship to (Hosy) and The National Center for Scientific Research (CNRS), Agence Nationale de la Recherche (DynHippo) to (Groc and Ferreira).
Words from the First Author
Memory is the most important ability that makes us human, but there is not much that is known about it. The loss of memory due to aging, illness, or accident can cause as much or even more grief than physical damage. It can also be difficult to live a positive life because of the sad memories of the past. Through the study of the molecular mechanisms of memory formation, I hope to realize a world where people can live even better life.
Publication Information
-
Hosokawa, T., Liu, P.W., Cai, Q., Ferreira, J.S., Levet, F., Butler, C., Sibarita, J.B., Choquet, D., Groc, L., Hosy, E., Zhang, M., & Hayashi, Y. (2021).
CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation. Nature neuroscience, 24(6), 777-785. [PubMed:33927400] [WorldCat] [DOI]